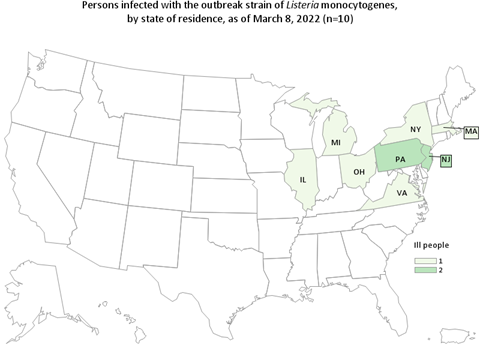
The FDA, along with CDC and state and local partners are investigating consumer complaints and/or reports of infant illness related to products from Abbott Nutrition’s Sturgis, MI facility. All of the ill patients are reported to have consumed powdered infant formula produced from Abbott Nutrition’s Sturgis, MI facility.
As of February 28, CDC has announced one additional illness of Cronobacter sakazakii with exposure to powdered infant formula produced at Abbott Nutrition’s Sturgis, MI facility. Cronobacter infection may have been a contributing cause of death for this patient. In total, this investigation includes four reports of Cronobacter sakazakii infections in infants (three from FDA complaints and one from a CDC case finding) and one complaint of a Salmonella Newport infection in an infant. All five (four Cronobacter infections and one Salmonella Newport infection) illnesses resulted in hospitalization and Cronobacter may have contributed to death in two patients.
The most recent patient was reported to have consumed Abbott Nutrition’s Similac PM 60/40 product with the lot code 27032K800 prior to Cronobacter sakazakii infection. FDA and CDC informed the firm of these findings and on February 28, 2022, Abbott Nutrition voluntarily recalled Similac PM 60/40 powdered infant formula with the lot code 27032K800. This is a specialty formula for certain infants who would benefit from lowered mineral intake and was not included in the previous recall. At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
This particular lot of Similac PM 60/40 was distributed to the U.S. and Israel. If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices.
We understand that infant formula is the sole source of nutrition for many infants and is an essential product. FDA is working with Abbott Nutrition to better assess the impacts of the recall and understand production capacity at other Abbott facilities that produce some of the impacted brands. We are also working with Abbott Nutrition on safe resumption of production at the Sturgis, MI facility. FDA is continuing to investigate and will update this advisory should additional consumer safety information become available.
Recommendation
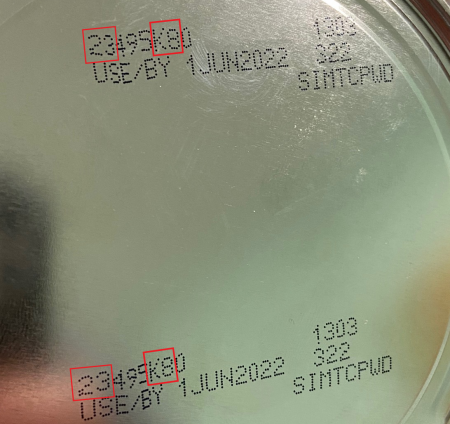
The FDA is advising consumers not to use recalled Similac, Alimentum, or EleCare powdered infant formulas. Recalled products can be identified by the 7 to 9 digit code and expiration date on the bottom of the package (see image below). Products are included in the recall if they have all three items below:
- the first two digits of the code are 22 through 37 and
- the code on the container contains K8, SH, or Z2, and
- the expiration date is 4-1-2022 (APR 2022) or later.
In addition to products described above, Abbott Nutrition has recalled Similac PM 60/40 with a lot code 27032K80 (can) / 27032K800 (case). At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
Additional recall information for the initial recall is available on the FDA website. Parents can also enter their product lot code on the company’s websiteExternal Link Disclaimer to check if it is part of the recall.
Additional Information for Parents and Caregivers:
The recalls do not include liquid formula products. Consumers should continue to use all product not included in the recalls.
Parents and caregivers should never dilute infant formula and should not make or feed homemade infant formula to infants. Consumers should also avoid purchasing imported formula through online sales, as it has the potential to be counterfeit.
If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices.
If you get infant formula through WIC, do not throw the formula out. Instead, you should take it to the store for a refund and exchange or call the company at 1-800-986-8540 to help you. WIC recipients should be able to obtain a different brand of similar formula. Call your local WIC clinic for more guidance. Also see:
More information on Cronobacter and infant formula is available on CDC’s website.
Recalled powdered infant formulas have the potential to be contaminated with Cronobacter, a bacterium that can cause severe foodborne illness primarily in infants. Cronobacter infections are rare but are especially high risk for newborn infants (see symptoms below).
Cronobacter bacteria can cause severe, life-threatening infections (sepsis) or meningitis (an inflammation of the membranes that protect the brain and spine). Symptoms of sepsis and meningitis may include poor feeding, irritability, temperature changes, jaundice (yellow skin and whites of the eyes), grunting breaths, and abnormal movements. Cronobacter infection may also cause bowel damage and may spread through the blood to other parts of the body.
If your child is experiencing any of these symptoms, you should notify your child’s healthcare provider and seek medical care for your child immediately. Healthcare providers and health departments are encouraged to report any confirmed cases of Cronobacter sakazakii to CDC.