* Baby food * Similac, Alimentum and EleCare from the ABBOTT brand
BVL
Possible contamination with Salmonella Newport and Cronobacter sakazakii
Reference to a public warning from the US Food and Drug Administration:
Possible contamination of US infant formula with Cronobacter sakazakii and Salmonella Newport
Status 03/24/2022
The US Food and Drug Administration (FDA) warns against the use of certain powdered infant formulas manufactured by Abbott Nutrition in Sturgis, Michigan.
On February 17, 2022, Abbott announced that it had initiated a voluntary recall of certain lots of potentially affected products. The products in question are Similac, Alimentum and EleCare powdered food, which are manufactured at the plant in Sturgis, Michigan.
The recall is related to consumer complaints about Cronobacter sakazakii and Salmonella Newport infections in the United States. In all of these cases, infant formula from the manufacturer Abbott Nutrition is said to have been consumed. All five cases related to these ailments required hospitalization. In two cases, the infection may have contributed to the deaths of the patients.
The FDA is warning against the purchase and consumption of certain infant formulas manufactured at Abbott Nutrition’s Sturgis, Michigan facility.
The products were shipped within the USA and in member states of the EU (Italy, Ireland, Croatia, Netherlands, Slovenia, Spain) and other third countries (e.g. Great Britain, India, Islamic Republic of Iran, Northern Ireland, Russian Federation, Turkey, Ukraine, United States of America ) expelled. According to current knowledge, the products mentioned were not sold in Germany.
There is a possibility that consumers could have purchased the products online via marketplaces or through short-term stays in the Member States or third countries.
The FDA advises consumers not to use Similac, Alimentum, or EleCare powdered infant formula if:
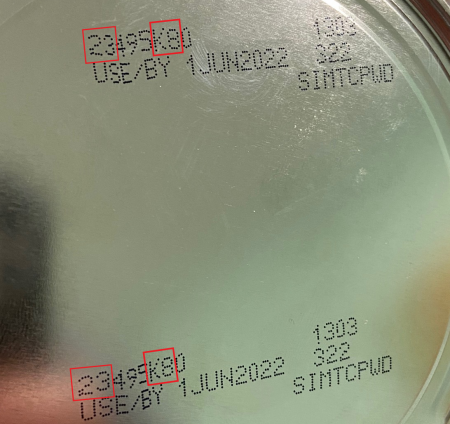
- the first two digits of the batch number begin with 22 to 37 and
- the details K8, SH or Z2 are included and
- a best-before date of April 1, 2022 is specified
The manufacturer offers the possibility to check the batch number of purchased products on the following page: https://www.similacrecall.com/us/en/product-lookup.html
For more information on the FDA’s warning, please visit the following link: https://www.fda.gov/news-events/press-announcements/fda-warns-consumers-not-use-certain-powdered-infant-formula-produced- Abbott Nutrition Facility
The manufacturer’s recall can be found at the following link: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/abbott-voluntarily-recalls-powder-formulas-manufactured-one-plant
For the warning in Turkish language, please refer to the following link: https://www.titck.gov.tr/duyuru/ilac-geri-cekme-24022022101035
Information on Salmonella and Cronobacter sakazakii
Salmonella and Cronobacter sakazakii can cause illness in infants when present in powdered infant formula. Although Cronobacter sakazakii and Salmonella cannot grow in powdered infant formula, they can survive for a long period of time and therefore pose a potential risk after liquid addition if the product is not heated sufficiently during preparation. Contamination of infant formula powder with Cronobacter sakazakiiand salmonella can cause serious illnesses in infants, such as diarrhea (sometimes bloody), fever, sepsis or meningitis, which can cause serious neurological and developmental problems and, in rare cases, can be fatal. Sepsis and meningitis can present with poor feeding, irritability, temperature changes, jaundice (yellow skin and whites of the eyes), and abnormal breathing and movements. Among infants, newborns (<28 days), especially preterm, low birth weight, or immunocompromised infants are most at risk of becoming infected.
(Unofficial translation of the information at: https://www.fsai.ie/news_centre/food_alerts/Similac.html )
Further information on salmonella, also in other languages, can be found here: http://www.infectionsschutz.de/erregersteckbriefe/salmonellen