Archives
-
Join 346 other subscribers
KSWFoodWorld
Blog Stats
- 438,606 Views
Category Archives: HPP
Research – Impact of High-Pressure Processing (HPP) on Listeria monocytogenes—An Overview of Challenges and Responses
High-pressure processing (HPP) is currently one of the leading methods of non-thermal food preservation as an alternative to traditional methods based on thermal processing. The application of HPP involves the simultaneous action of a combination of several factors—pressure values (100–600 MPa), time of operation (a few–several minutes), and temperature of operation (room temperature or lower)—using a liquid medium responsible for pressure transfer. The combination of these three factors results in the inactivation of microorganisms, thus extending food shelf life and improving the food’s microbiological safety. HPP can provide high value for the sensory and quality characteristics of products and reduce the population of pathogenic microorganisms such as L. monocytogenes to the required safety level. Nevertheless, the technology is not without impact on the cellular response of pathogens. L. monocytogenes cells surviving the HPP treatment may have multiple damages, which may impact the activation of mechanisms involved in the repair of cellular damage, increased virulence, or antibiotic resistance, as well as an increased expression of genes encoding pathogenicity and antibiotic resistance. This review has demonstrated that HPP is a technology that can reduce L. monocytogenes cells to below detection levels, thus indicating the potential to provide the desired level of safety. However, problems have been noted related to the possibilities of cell recovery during storage and changes in virulence and antibiotic resistance due to the activation of gene expression mechanisms, and the lack of a sufficient number of studies explaining these changes has been reported.
Research – Foodborne pathogen inactivation in fruit juices utilizing commercial scale high-pressure processing: Effects of acidulants and pH
The effects of juice pH, type of acidulant, and post-treatment refrigeration on the high-pressure processing (HPP) inactivation of Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes in acid beverages were evaluated. Inoculated apple, orange, and grape juices (at their original pH and adjusted to pH 4.00, 4.50, and 5.00) were treated at 550 MPa for 1 min at 5 °C. In addition, inoculated model solutions acidified to a pH of 5.00 with acetic, citric, malic, and tartaric acids were treated at 400 MPa for 1 min at 5 °C. The effect of refrigerated storage for 24 h after treatment on pathogen inactivation in both experiments was also assessed. A greater than 5-log reduction of the three pathogens inoculated was achieved in all juices immediately after HPP at the juices’ original pH, and of L. monocytogenes under all experimental conditions. Refrigerated storage for 24 h after HPP treatment improved the inactivation of E. coli O157:H7, to >5-log reduction, at pH 4.00 in apple juice and of Salmonella in the three juices at pH 4.00. The type of acidulant did not significantly ( p > 0.01) affect E. coli or Salmonella inactivation in acidified model solutions but a greater than 5-log reduction after HPP was only achieved for L. monocytogenes when acetic acid was used. The effectiveness of HPP for pathogen inactivation depended largely on product pH and the target pathogen of concern.
Research – Pilot Scale Assessment of High-Pressure Processing (HPP) to Enhance Microbiological Quality and Shelf Life of Fresh Ready-to-Eat (RTE) Blue Crab Meat
Abstract
Blue crab (Callinectes sapidus) is a highly valuable wild fishery species of crab native to the waters of the western Atlantic Ocean and the Gulf of Mexico. The annual commercial production of live blue crabs is approximately 50,000 metric tons with a dockside value of USD 200 million. Presently the US blue crab processing industry sells crab meat in three basic forms: fresh crab meat, pasteurized crab meat, and frozen crab meat. By far “Fresh” is the most desirable form of crab meat. However, fresh crab meat has a limited shelf life. This study evaluated the effects of high-pressure processing (HPP) on enhancing the microbiological quality and shelf life of blue crab meat. Live blue crabs were pressure-cooked in a retort (≥115 °C for 4–6 min). The crab meat was handpicked, packed in plastic containers with seals, subjected to HPP treatment, and stored at 4 °C. Container integrity and water leakage issues were examined by observation in addition to weight comparison before and after HPP treatment; the shelf life of crab meat with and without HPP treatments was examined via microbiological tests and sensory evaluations. Results show that polypropylene containers sealed with 10K OTR (oxygen transmission rate) film could withstand high pressure without water leakage issues; HPP treatment at 600 MPa for 3 min could extend the shelf life of fresh, cooked, and handpicked crab meat from 6 days to 18 days based on the strictest APC (aerobic plate account) limit (APC ≤ 100,000 CFU/g). The sensory quality of the HPP-treated crab meat was well accepted throughout the 3-week storage period. The results support the use of HPP as an effective non-thermal processing technology to enhance the microbiological quality and extend the shelf life of fresh RTE blue crab meat.
Posted in Food Microbiology Research, HPP, Research, Technology
Research – High-Pressure Inactivation of Bacillus cereus in Human Breast Milk
Abstract
Although Holder pasteurization is the recommended method for processing breast milk, it does affect some of its nutritional and biological properties and is ineffective at inactivating spores. The aim of this study was to find and validate an alternative methodology for processing breast milk to increase its availability for newborn babies and reduce the financial loss associated with discarding milk that has become microbiologically positive. We prepared two series of breast milk samples inoculated with the Bacillus cereus (B. cereus) strain to verify the effectiveness of two high-pressure treatments: (1) 350 MPa/5 min/38 °C in four cycles and (2) cumulative pressure of 350 MPa/20 min/38 °C. We found that the use of pressure in cycles was statistically more effective than cumulative pressure. It reduced the number of spores by three to four orders of magnitude. We verified that the method was reproducible. The routine use of this method could lead to an increased availability of milk for newborn babies, and at the same time, reduce the amount of wasted milk. In addition, high-pressure treatment preserves the nutritional quality of milk.
Posted in Food Microbiology Research, High Pressure, HPP, Research
Research – Inactivation Kinetics of Foodborne Pathogens in Carrot Juice by High-Pressure Processing
Abstract
In this study, Salmonella Typhimurium, Escherichia coli, and Listeria monocytogenes were separately inoculated in sterilized carrot juice and subjected to various types of high-pressure processing (HPP) at 200–600 MPa for 0.1–15 min to observe the effects of HPP on the inactivation kinetics of foodborne pathogens in carrot juice. The first-order model fits the destruction kinetics of high pressure on foodborne pathogens during the pressure hold period. An increase in pressure from 200 to 600 MPa decreased the decimal reduction time (D values) of S. Typhimurium, E. coli, and L. monocytogenes. Under pressure ≥ 400 MPa, the D values of E. coli were significantly higher than those of S. Typhimurium and L. monocytogenes, indicating that E. coli had greater resistance to high pressures than the others. The Zp values (the pressure range that causes the D values to change by 90%) of E. coli, S. Typhimurium, and L. monocytogenes were 195, 175, and 170 MPa, respectively. These results indicated that L. monocytogenes and E. coli were the most and least sensitive, respectively, to pressure changes. Additionally, the three bacteria were separately inoculated into thermal-sterilized carrot juice and subjected to 200–600 MPa HPP for 3 min. The treated carrot juices were stored at 4 °C for 27 d. Following S. Typhimurium and E. coli inoculation, the bacterial counts of the control and 200 MPa treatments remained the same during the storage duration. However, they decreased for the 300 and 400 MPa treatment groups with increasing storage duration. During the storage period, no bacterial growth was observed in the 500 and 600 MPa treatments. However, the bacterial number for the control and pressure treatment groups increased with prolonged storage duration following inoculation with L. monocytogenes. Therefore, following HPP, residual L. monocytogenes continued growing stably at low temperatures. Overall, HPP could inhibit and delay the growth of S. Typhimurium and E. coli in carrot juice during cold storage, but it was ineffective at inhibiting the growth of L. monocytogenes. There was a risk of foodborne illness despite the low-temperature storage of juice. The innovation of this preliminary study is to find the impact of high pressure on the inactivate kinetics of three food pathogens in carrot juice and its practical application in simulated contaminated juice.
Research – High-Pressure Processing—Impacts on the Virulence and Antibiotic Resistance of Listeria monocytogenes Isolated from Food and Food Processing Environments
Abstract
High-pressure processing (HPP) is one of the non-thermal methods of food preservation considered to be safe but may cause an increase/decrease in virulence potential and antibiotic resistance. The aim of the present study was to evaluate the survival of L. monocytogenes isolates after high-pressure processing (200 and 400 MPa for 5 min) and to determine changes in phenotypic and genotypic antibiotic resistance and virulence after this treatment. The 400 MPa treatment was shown to be effective in reducing pathogens to safe levels; however, the potential for cell recovery during storage was observed. In addition, studies on changes in virulence indicated possibilities related to a decrease in actA gene expression, overexpression of the hly and osfX gene, and an increase in biofilm-forming ability. The studies on changes in antibiotic resistance of isolates showed that all isolates showing initial susceptibility to lincomycin, fosfomycin, trimethoprim/sulfamethoxazole, and tetracycline became resistant to these antibiotics, which was associated with an increase in the values of minimum inhibitory concentrations. An increase in the expression of antibiotic resistance genes (mainly tetA_1, tetA_3, tetC) was also observed (mainly after the application of 200 MPa pressure), which was isolate dependent. However, it is noteworthy that the induced changes were permanent, i.e., they persisted even after the restoration of optimal environmental conditions. The results presented in our work indicate that the stress occurring during HPP can affect both phenotypic and genotypic changes in the virulence and antibiotic resistance potential of pathogens isolated from food and food processing environments. The potential associated with cell recovery and persistence of changes may influence the spread of virulent isolates of pathogens with increased antibiotic resistance in the food and food processing environment.
Research – Microbiological, Physicochemical and Nutritional Properties of Fresh Cow Milk Treated with Industrial High-Pressure Processing (HPP) during Storage
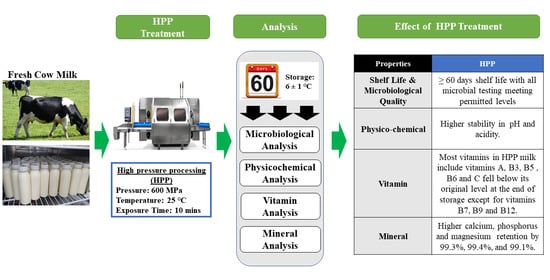
Abstract
The safety, shelf life, and quality of fresh cow milk treated using industrial High-Pressure Processing (HPP) treatment at 600 MPa for 10 min was studied to identify the novelty of this non-thermal technology in milk processing. Changes in microbiological and physicochemical properties, including nutritional values of vitamins and amino acid profiles, were measured for a 60-day storage period at 6 °C +/− 1 °C. The HPP treatment produced milk that met all microbial safety requirements and exhibited a shelf life beyond 60 days in a hot and humid region. High physicochemical stability was achieved, with consistent pH and undetectable titratable acidity. The HPP treatment successfully retained all vitamins and minerals, including calcium (99.3%), phosphorus (99.4%), and magnesium (99.1%). However, the 60-day storage caused some degradation of Vitamin A (25%), B3 (91%), B5 (35%), B6 (80%), and C (85%), and minerals, including potassium (5%) and zinc (18%) when compared with fresh milk. This research has shown that the adoption of advanced treatment with HPP is very beneficial to the dairy industry in preserving milk quality in terms of its physicochemical and nutritional properties and extending its storage shelf life beyond 60 days.
Research – The Effect of High-Pressure Processing on the Survival of Non-O157 Shiga Toxin-Producing Escherichia coli in Steak Tartare: The Good- or Best-Case Scenario?
Abstract
Samples of steak tartare were artificially contaminated with a cocktail of Shiga toxin-producing Escherichia coli (STEC) O91, O146, O153, and O156 to the level of 3 log and 6 log CFU/g. Immediately after vacuum packing, high-pressure processing (HPP) was performed at 400 or 600 MPa/5 min. Some of the samples not treated with HPP were cooked under conditions of 55 °C for 1, 3, or 6 h. HPP of 400 MPa/5 min resulted in a 1–2 log reduction in the STEC count. In contrast, HPP of 600 MPa/5 min led to the elimination of STEC even when inoculated to 6 log CFU/g. Nevertheless, sub-lethally damaged cells were resuscitated after enrichment, and STEC was observed in all samples regardless of the pressure used. STEC was not detected in the samples cooked in a 55 °C water bath for 6 h, even after enrichment. Unfortunately, the temperature of 55 °C negatively affected the texture of the steak tartare. Further experiments are necessary to find an optimal treatment for steak tartare to assure its food safety while preserving the character and quality of this attractive product.
Posted in Decontamination Microbial, E.coli O157, E.coli O157:H7, Food Micro Blog, Food Microbiology, Food Microbiology Blog, Food Microbiology Testing, HPP, microbial contamination, Microbial growth, Microbiological Risk Assessment, Microbiology, Microbiology Investigations, Microbiology Risk, O146, O153, O156, STEC, STEC E.coli
Research – The effectiveness and safety of high pressure food treatment
Definition and applicable regulations
High pressure treatment (HPP), also known as “high pressure hydrostatic treatment (HHP)” or “ultra pressure treatment (UHP)”, is a non-thermal (<45 °C) food preservation technology that inactivates forms vegetative pathogens and spoilage microorganisms – does not inactivate bacterial spores – using high pressures with minimal effects on taste, texture, appearance or nutritional value.
High pressure treatment is not specifically regulated within the EU, however, according to Regulation (EC) 852/2004 regarding the hygiene of food products, HPP is considered a processing. Any relevant food safety legislation is applicable to HPP ─hygiene requirements, microbiological criteria, food contact materials, traceability and labeling requirements. The guidance document on the application of certain provisions of Regulation (EC) 852/2004 introduces, in section 9.6, clarifications on the implementation of the HPP.
The European Food Safety Authority (EFSA) was asked to issue a scientific opinion on the efficacy (reduction of foodborne pathogen levels) and food safety of HPP. Specifically, the terms of reference of the mandate required: to provide an overview of the foods to which the HPP is or could be applied, together with the processing conditions; list the intrinsic and extrinsic factors that may influence the effectiveness of HPP; and assess the potential chemical and microbiological risks to food safety of HPP-treated foods compared to untreated foods or foods that are routinely applied in order to increase their microbiological food safety.An evaluation of the use of HPP was also requested for two specific purposes: as an alternative to the pasteurization and ultra high temperature (UHT) treatment of raw milk and raw colostrum; and for the control of Listeria monocytogenes in ready-to-eat foods. Quality aspects and organoleptic properties were beyond the scope of this mandate.
Type of food treated and processing conditions
Virtually all types of food can be treated with HPP. However, foods that contain trapped air (eg, bread, cakes, whole and freshly cut fruits and vegetables) are not suitable for HPP because their porous structure will be adversely affected. Low-moisture foods, such as powdered products and nuts, are not usually treated with this technology due to low microbial inactivation when the water content is less than 40%.
According to the data collected through a questionnaire in food operators, the relative importance between the types of food that are currently treated with HPP is as follows:
- High – Cooked meat products (including sliced, hot dog , etc.) ready-to-eat, raw-cured meat products (fermented or dried); acidic fruit and vegetable juices, guacamole and ready-to-eat pre-cooked meals.
- Medium – Fruit purees, moist salads (pH <5), and other spreads (eg, hummus, pesto); crustaceans, shellfish, molluscs and derived products; baby food.
- Low : fish and fishery products; milk, raw milk and pasteurized cheese, processed cheese in sauce or spread and dairy products (except cheese).
In the industrial context, to inactivate microorganisms, pressures of between 400 and 600 MPa are applied, for 1.5 to 6 minutes. The water used as a pressure transmission fluid for HPP is often pre-cooled to 4-8 °C.
Typically, products (liquid, semi-solid and solid foods) are packaged in flexible plastic materials prior to HPP to prevent recontamination of the product after HPP. Equipment for processing liquids in bulk before packaging is also available, but is currently rarely used.
Intrinsic and extrinsic factors of food that influence the effectiveness of high pressure treatment
According to EFSA’s scientific opinion, the main intrinsic factors in food that influence the effectiveness of HPP in terms of reducing vegetative microorganisms are water activity (a w ) and pH. Microbial inactivation increases with high values of a w and low pH values. Carbohydrates, proteins and lipids have a protective effect on microorganisms, which reduces microbial reduction. The main extrinsic factors are pressure and target pressure retention time. The type of microorganism, the taxonomic unit and the strain and the physiological state of the microorganisms to be inactivated also affect the effectiveness of the HPP.
The efficacy of HPP in different food matrices is variable due to the interactions between specific intrinsic factors, which makes it difficult to predict the efficacy of HPP in a complex food matrix.
EFSA recommends that the interactions of the different components be considered in the planning of the assessment of the impact of intrinsic factors on the effectiveness of PPPs and that validation studies be performed on actual food matrices.
Possible chemical and microbiological hazards associated with high pressure treatment
Food HPP poses no additional microbiological risk to food safety (eg, spore activation, induction of sublethal cell damage, conversion of normal form of prions to amyloid forms, and induction of virulence, gene expression for toxins and cross-resistance to other stresses) compared to other treatments commonly applied to these foods (eg, thermal pasteurization).
EFSA has also assessed the risk associated with mycotoxins and chemical processing contaminants by establishing that PPH-treated foods do not present a higher risk compared to conventionally treated foods.
HPP does not generate additional chemical food safety hazards from food contact materials compared to foods treated under similar temperature and weather conditions without HPP.
High pressure treatment as an alternative to pasteurization of milk
When raw milk, colostrum, dairy products or colostrum products are subjected to a heat treatment, such as pasteurisation or ultra-high temperature (UHT) treatment, the treatment must comply with the conditions laid down in the Regulation (CE) 853/2004. According to this Regulation, if pasteurization is used for these products, food operators must ensure that the following specifications are met: a high temperature for a short period of time (at least 72 ° C for 15 seconds), a low temperature for a long period of time (at least 63 °C for 30 minutes), or any other combination of temperature and time conditions to obtain an equivalent effect.
There is a growing interest in the use of HPP as an alternative treatment to pasteurization and UHT because it is expected to maintain properties closer to those of raw milk and colostrum.
According to the data collected and evaluated by EFSA, it was determined that HPP could not achieve logarithmic reductions (log 10 ) equivalent to those achieved by thermal pasteurization of milk (more than 10 log 10 ) or by UHT (log 10). more than 12 log 10 ). However, HPP conditions could be identified to achieve reductions equivalent to those recommended by international agencies as benchmarks of performance criteria for pasteurization (eg, reductions of 5, 6, 7, and 8 log 10 ). ).From the mathematical models obtained, several examples are provided of the minimum requirements (combination of pressure and time) of the HPP that, with a high certainty, would allow to reach the different criteria of operation.
Under the most stringent industrially used HPP conditions (600 MPa for 6 minutes), reductions of 5 log 10 for Mycobacterium bovis , 8 log 10 for Shiga toxin-producing Escherichia coli (ECTS or STEC),Listeria monocytogenes , Salmonella spp . and Campylobacter spp. , and 6 log 10 for Staphyloccoccus aureus .
According to EFSA, no data were found on the impact of HPP on the reduction of Brucella melitensis and tick-borne encephalitis virus (TBEV) and therefore no conclusions could be drawn for these. dangers.
EFSA evaluated several milk and colostrum compounds to determine their suitability as indicators of HPP efficacy, including the endogenous alkaline milk phosphatase enzyme (ALP) – widely used to verify the proper thermal pasteurization of milk. γ-glutamyltransferase (GGT), xanthine oxidase (XoX), β-lactoglobulin (β-Lg) or lactoferrin (LF).
In view of the available evidence, EFSA concludes that none of the evaluated indicators can currently be proposed as an appropriate indicator for use under the commercially viable technological conditions of HPP applied to industry (400 and 600 MPa for 1.5-6 minutes) and recommends further in-depth studies to determine the suitability of such compounds as indicators of HPP efficacy.
Efficacy of high pressure treatment for the control of Listeria monocytogenes in ready-to-eat foods
The most relevant foods associated with human listeriosis in the EU that are also relevant to be treated with HPP to increase microbiological food safety include ready-to-eat cooked meat products, soft and semi-soft cheeses, fresh cheeses and smoked or marinated fish. .
For ready-to-eat cooked meat products, the minimum requirements (combinations of pressure and retention time) were derived, which would achieve reductions of 1 to 5 log 10 for L. monocytogenes . For the other types of ready-to-eat foods relevant to listeriosis, the high uncertainty of the data did not allow the establishment of generic minimum HPP conditions, so specific validation studies are required for each specific product.
Salmonella spp. and E. coli were identified as the most relevant additional hazards, apart from L. monocytogenes, in ready-to-eat foods associated with human listeriosis. In the foods mentioned, these pathogens ( Salmonella and E. coli ) are generally more sensitive to HPP than L. monocytogenes and are thought to be inactivated to a similar or greater extent.
According to the EFSA report, further studies on the inactivation by HPP of L. monocytogenes and other pathogenic bacteria relevant to ready-to-eat foods, such as smoked fish, marinated fish, soft and semi-soft cheese, would be needed to establish the generic minimum requirements for HPP to ensure the safety of these foods.
USA – Microbiological Surveillance Sampling: FY17–19 Processed Avocado and Guacamole
The U.S. Food and Drug Administration collected and tested processed avocado, the main ingredient in guacamole, and finished guacamole as part of the agency’s proactive and preventive approach to deploying its sampling resources with the ultimate goal of preventing contaminated food from reaching consumers.
Assignment Overview
The assignment began in November 2017 and ended in September 2019. In total, the FDA collected and tested 887 samples of processed avocado and guacamole (domestic and imported product) for Salmonella spp. and Listeria monocytogenes. This total is smaller than the initial number of samples the agency set out to collect and test because the agency encountered factors that twice required a reduction of the collection target, as explained in the Sample Collection section of this report (page 6).
As to the design of the assignment, the FDA directed its field staff not to collect products that had undergone high-pressure processing (HPP) or products intended for HPP. HPP is a “kill step” validated to eliminate pathogenic microorganisms in food, and it is often used in the manufacture of processed avocado and guacamole. In seeking to exclude from the assignment products that had been HPP-treated, the FDA’s intent was to focus on products that posed the greatest risk to consumers.
The agency learned during its evaluation of the test results that some of the products collected had received HPP treatment but were not labeled as such. FDA staff worked retrospectively with industry to identify the HPP-treatment status of the samples collected but could not determine the status of a number of samples. Those samples were designated as “could not ascertain” for purposes of the data analysis.
Findings and Follow-up Actions
The FDA detected Salmonella spp.in two samples which were later determined to be distinct samples of the same brand of domestically manufactured guacamole from different lots. Neither sample had received HPP treatment. In addition, the agency detected Listeria monocytogenes in 15 samples from nine different firms. Of those 15 samples, eight had not been HPP treated. The HPP-treatment status of the other seven samples could not be ascertained.
When the FDA detected a pathogen in a domestic sample, agency personnel worked with the company that owned or distributed the affected product to conduct a voluntary recall in all cases in which product was available, or likely to still be available, to consumers. The FDA also conducted one follow-up inspection of a domestic facility, and state officials in Florida likewise conducted one domestic inspection. As to the imported samples, the agency refused to admit lots associated with the positives and placed the responsible companies on import alert. In all, the agency placed two firms on import alert. In addition, the agency conducted whole genome sequencing (WGS) analysis on the positives but was unable to determine whether processed avocado or guacamole were the food vehicle associated with any known human illnesses.
In addition to affirming that Salmonella spp. and Listeria monocytogenes may be present in processed avocado and/or guacamole, the assignment data show that the estimated prevalence of these pathogens in the non-HPP-treated samples was higher than in the HPP-treated samples. This finding appears to support other research that shows HPP is effective at neutralizing pathogenic microorganisms,[1] even as this assignment was not designed to compare possible differences based on HPP-treatment status. The findings also underscore the need for processors and others in the processed avocado and guacamole supply chain to comply with the FDA’s Preventive Controls for Human Food Rule[2] and for importers of these foods to comply with the FDA’s Foreign Supplier Verification Programs Rule.[3]
Posted in Decontamination Microbial, FDA, Food Micro Blog, Food Microbiology, Food Microbiology Blog, Food Microbiology Research, Food Microbiology Testing, HPP, Listeria, Listeria monocytogenes, microbial contamination, Microbiological Risk Assessment, Microbiology, Microbiology Investigations, Research, Salmonella

