What is BS 8580-2 – Risk assessments for Pseudomonas aeruginosa about?
Our experts identified a lack of guidance on how to conduct risk assessments for Pseudomonas aeruginosa (PA) and other opportunistic waterborne pathogens other than Legionella. To fill that gap, BS 8580-2 is a new British Standard recommending a PA risk assessment process and supplying information and support on how to understand microbial hazards, prioritize actions and minimize risks.
Who is BS 8580-2 – Risk assessments for Pseudomonas aeruginosa for?
BS 8580-2 on risk assessments for pseudomonas aeruginosa applies in all types of healthcare provision, including hospitals, and care, nursing and residential homes, together with other settings where water systems and associated equipment can pose a risk. This can include in the educational, travel, industrial, leisure and beauty sectors, including health spas, nail bars and tattoo parlours.
Users of BS 8580-2 will be building and design engineers and architects; providers of fittings, outlets and components for water systems; installers and commissioners; risk assessors; regulatory bodies; building services engineers; water treatment consultants; travel, leisure and other relevant buildings owners and operators; and those responsible for the safe management of water systems, especially within leisure centres, schools, swimming pools, passenger vessels, spa pools, hot tubs etc.
BS 8580-2 will also interest clinicians, microbiologists, augmented care specialists and infection controllers in healthcare.
What does BS 8580-2 – Risk assessments for Pseudomonas aeruginosa cover?
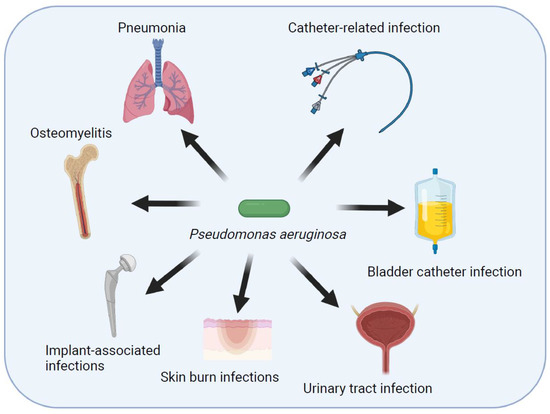
BS 8580-2 gives recommendations and guidance on how to carry out risk assessments for pseudomonas aeruginosa (PA) and other waterborne pathogens whose natural habitat is within constructed water systems and the aqueous environment (autochthonous), rather than those present as a result of a contamination event. It includes those pathogens that can colonize and grow within water systems and the associated environment.
BS 8580-2 also covers risk assessments of distributed water systems and associated equipment, system components and fittings as well as above ground drainage systems. It covers PA risk assessment reviews and reassessments where a previous assessment has been undertaken and risk factors identified. It takes account of all relevant environmental and clinical factors and aspects of human behaviour leading to contamination events. It considers risk factors within the associated environment leading to conditions which can encourage the colonization and growth of waterborne pathogens and transfer of antibiotic resistance.
NOTE: BS 8580-2 does not cover risk assessments for Legionella spp.; these are covered in BS 8580-1, or risk assessments for enteric microorganisms derived from human or animal faecal contamination or sewage ingress.
Why should you use BS 8580-2 – Risk assessments for Pseudomonas aeruginosa?
You should use BS 8580-2 on risk assessments for pseudomonas aeruginosa because:
- It plugs an information gap in relation to pseudomonas aeruginosa (PA) risk assessments, taking its unique additional considerations into account
- It codifies the latest and most efficient approach to multidisciplinary PA risk assessments
- Its recommended processes can be applied to other opportunistic waterborne pathogens
- It can help healthcare providers demonstrate compliant risk management in respect of Dept of Health Guidance
- It can help leisure sector organizations maintain compliance with their legal health and safety obligations
- It can help users develop their expertise in risk assessment and strengthen organizational risk management
BS 8580-2 contributes to UN Sustainable Development Goal 3 on good health and well-being and Goal 6 on clean water and sanitation.