The FDA and CDC, in collaboration with state and local partners, investigated illnesses in a multistate outbreak of E. coli O157:H7. As of January 6, 2022, CDC declared this outbreak over.
FDA traced the supply chain for this positive product sample and deployed investigators to three farms in two separate states: California and Oregon. FDA conducted inspections, including sample collection and analysis, but inspections were limited because at the time the fields were fallow and no production activities were being conducted for spinach at any of the three farms. All samples collected were reported negative and no source or direct routes of contamination to the suspected spinach were found during the inspections. Investigators did report that one location processes product and sometimes uses product from multiple growers in one production run. This type of product co-mingling is not uncommon; but continues to present challenges for traceback investigations of foodborne outbreaks.
Recommendation
CDC announced the outbreak is over. There are no recommendations for consumers, retailers, or suppliers.
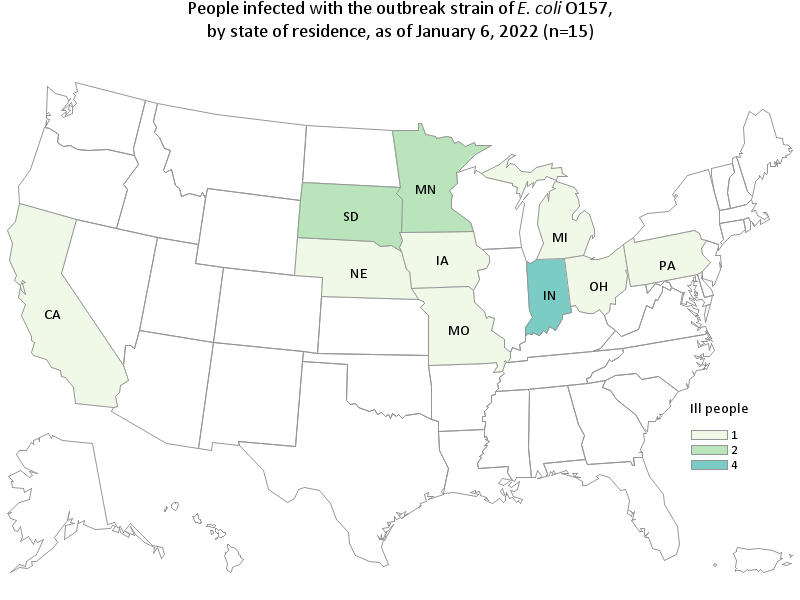
Case Count Map Provided by CDC
Case Counts
Total Illnesses: 15
Hospitalizations: 4
Deaths: 0
Last Illness Onset: November 8, 2021
States with Cases: CA (1), IA (1), IN (4), MI (1), MN (2), MO (1), NE (1), OH (1), PA (1) SD (2)