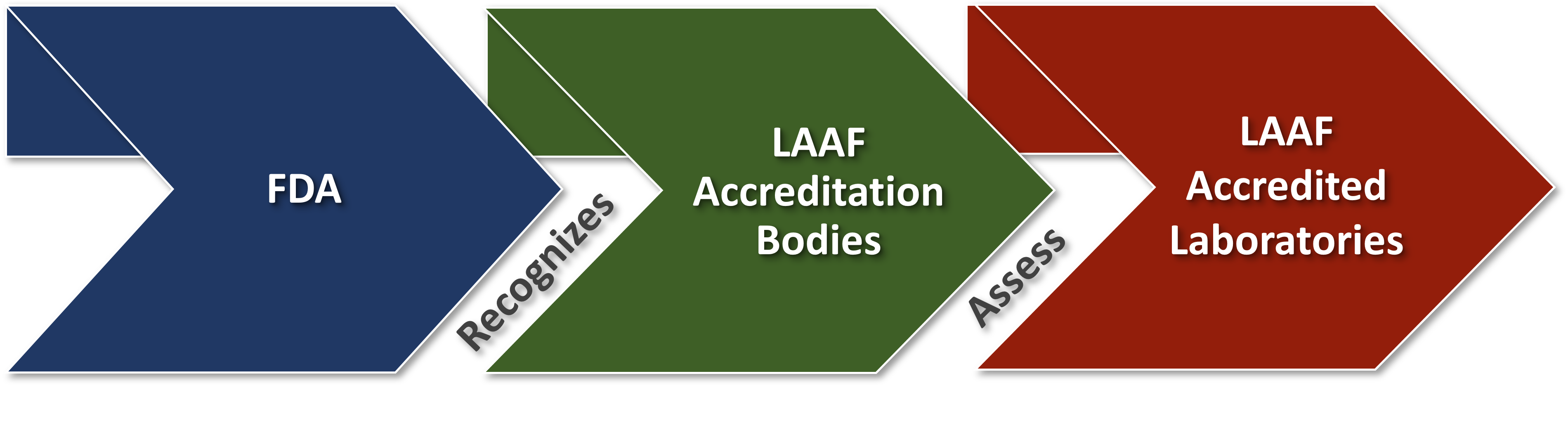
The FDA Food Safety Modernization Act (FSMA) final rule on Laboratory Accreditation for Analyses of Foods (LAAF) establishes a laboratory accreditation program for the testing of food in certain circumstances. Under the LAAF program, FDA will recognize accreditation bodies (ABs) that will accredit laboratories to the standards established in the final rule (referred to as LAAF-accredited laboratories).
The final rule specifies eligibility requirements that ABs and laboratories wishing to participate in the program will need to satisfy, as well as procedures for how the FDA will manage and oversee the program. In certain circumstances, owners and consignees will be required to use a LAAF-accredited laboratory for food testing. FDA will maintain an online public registry listing recognized accredited bodies and LAAF-accredited laboratories.
The establishment of the LAAF program is intended to improve the accuracy and reliability of certain food testing through the uniformity of standards and enhanced FDA oversight of participating laboratories.
Enlarge in PDF (241KB)